


Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promotes tumor cell extravasation
Michelle B. Chen, Cathy Yu, David C. Benjamin, Hesham Azizgolshani, Richard O. Hynes, Roger D. Kamm
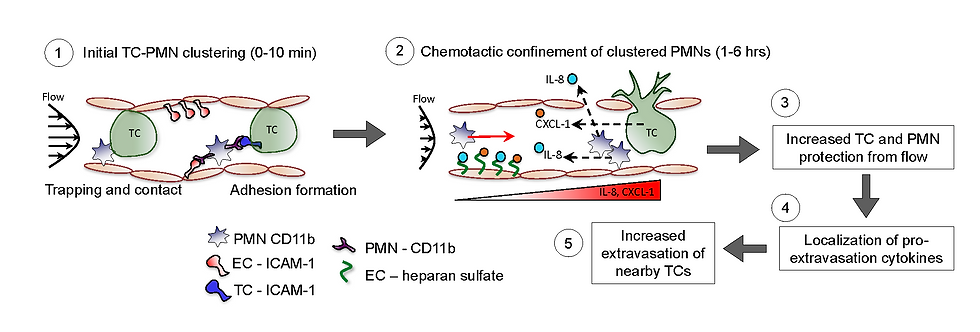
Metastasis is the cause of the vast majority of cancer-related mortality and represents an urgent clinical need. It has long been clear that tumor cells require the assistance of cells from the innate immune system, including platelets, neutrophils, and monocytes/macrophages in order to successfully metastasize. In particular, neutrophils have been shown to be rapidly recruited to arrested tumor cells and promote tumor cell extravasation. However, it is not entirely clear how these two cell types are interacting to promote tumor cell extravasation.
Roger Kamm's lab in the department of mechanical engineering at MIT has developed a microfluidic model of human vasculature that can be used to study the extravasation of tumor cells. Tumor cells and neutrophils can be co-infused into this system to study their interactions during extravasation.
They have found that when tumor cells and neutrophils are co-infused into this system, neutrophils associated with arrested tumor cells will migrate but remain nearby while neutrophils far from arrested tumor cells migrate randomly. This confined migration is dependent on tumor cell-derived CXCL-1 and neutrophil-derived IL-8. Blocking either of these chemokines allows neutrophils to migrate away from arrested tumor cells and inhibits tumor cell extravasation.
We validated that these results by injecting human tumor cells and human neutrophils into 2-day-old zebrafish embryos. We first confirmed that human neutrophils enhance human tumor cell extravasation in zebrafish embryos. We then imaged their interactions over the course of 6 hours and confirmed that tumor cell cluster-associated neutrophils were more confined in their migration compared to free neutrophils.